Ensuring drug quality and patient safety is at the heart of pharmaceutical manufacturing, where strict regulatory frameworks like FDA 21 CFR Part 11 and ISO 17025 are non-negotiable. As operations scale and complexity increase, so does the need for robust digital infrastructure. A Pharmaceutical Laboratory Information Management System (LIMS) has become indispensable in this environment, offering automation, data integrity, and end-to-end compliance. By streamlining quality control and providing real-time oversight, LIMS empowers pharmaceutical companies to meet regulatory demands efficiently and consistently. In this blog, we explore how Pharmaceutical LIMS enhances drug safety, outline best practices for implementation, and highlight the essential features to look for in an effective solution.
Understanding Pharmaceutical LIMS and Its Role in Drug Safety
What is Pharmaceutical LIMS?
A Laboratory Information Management System (LIMS) is far more than just lab software; it’s a foundational tool that supports operational excellence and regulatory compliance in modern pharmaceutical manufacturing. At its core, LIMS is designed to streamline laboratory workflows, centralize sample data, and automate routine tasks, enabling pharmaceutical companies to deliver safe, effective products with greater speed and confidence.
In the highly regulated pharmaceutical industry, where every data point matters and every deviation carries risk, LIMS acts as a digital backbone for quality assurance. It ensures compliance with global regulatory authorities such as the FDA, EMA, and guidelines under GxP (Good Laboratory, Manufacturing, and Clinical Practices). By maintaining meticulous records and automating critical processes, a pharmaceutical LIMS enhances traceability, minimizes human error, and drives consistent product quality.
Why Drug Quality and Safety Depend on LIMS
Pharmaceutical laboratories operate in a high-stakes environment. From raw material testing to final product release, a single misstep, such as mislabeling a sample, using an uncalibrated instrument, or entering incorrect data, can compromise patient safety and trigger costly regulatory actions. A robust LIMS addresses these vulnerabilities head-on by embedding control and consistency into every phase of lab operations.
Key Functions That Safeguard Drug Quality
Best Practices for Leveraging Pharmaceutical LIMS for Drug Quality
Successfully implementing a Pharmaceutical LIMS involves more than just installing software, it requires a strategic approach to automation, compliance, and configuration. The true value of a LIMS is realized when it becomes deeply embedded in laboratory workflows, supporting both daily operations and long-term regulatory obligations. Below are best practices to help pharmaceutical organizations maximize the impact of their LIMS in ensuring drug quality and patient safety.
Automating Laboratory Workflows for Greater Efficiency and Accuracy
Automation is at the heart of a modern pharmaceutical LIMS. From sample intake to certificate generation, LIMS can streamline complex lab processes and eliminate time-consuming manual steps. By automating sample management, labs can track every sample’s journey from receipt and preparation, through testing and approval, to storage or disposal with precise timestamps and traceability. This reduces the risk of misplaced samples and improves overall accountability.
Automated reporting and data entry features reduce the chances of transcription errors and enable quicker turnaround times. Rather than relying on spreadsheets or paper forms, lab staff can enter or import results directly into the system, often with the help of instrument integration. This speeds up result validation and ensures that calculations and limit checks are performed consistently and accurately.
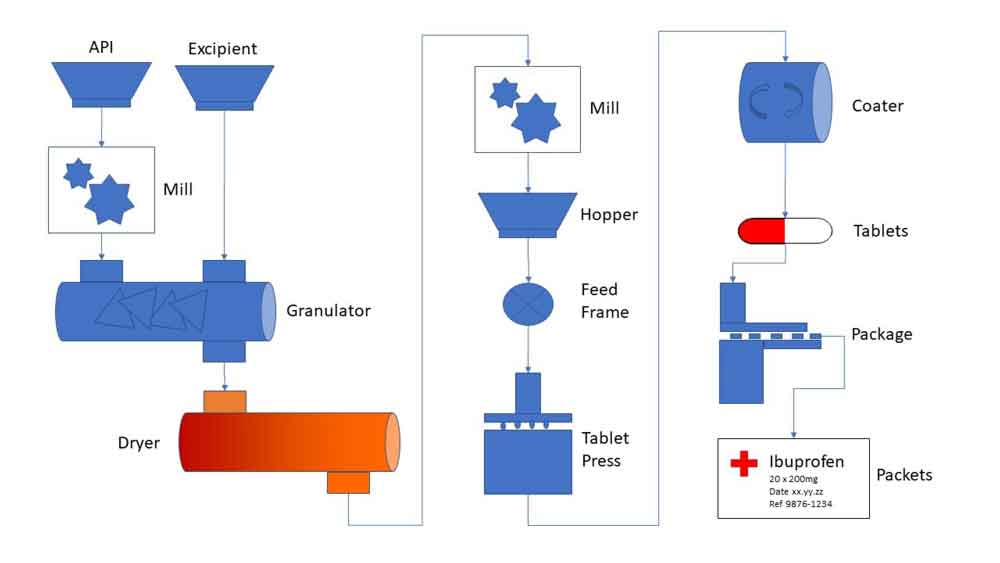
Batch traceability is another critical benefit. A pharmaceutical LIMS allows users to track raw materials, intermediate products, and finished goods throughout the manufacturing process. Comprehensive audit trails capture every interaction with the data who accessed it, what was changed, when, and why, ensuring full transparency during audits or investigations. Laboratory information management systems like Xybion LIMS exemplify this level of automation and traceability, making them powerful tools for regulated pharmaceutical environments.
Ensuring Data Integrity and Maintaining Regulatory Compliance
Data integrity is foundational in regulated environments. A Pharmaceutical LIMS should be designed to support the ALCOA+ principles assuring that all data is Attributable, Legible, Contemporaneous, Original, and Accurate, as well as Complete, Consistent, Enduring, and Available. These principles ensure that every piece of information captured is trustworthy, reproducible, and aligned with regulatory expectations.
To fully support FDA and EMA compliance, the system should include capabilities such as electronic signatures, automated audit logs, and user-specific access controls. Pharmaceutical LIMS platforms such as Xybion LIMS help enforce these standards by embedding compliance checks into each workflow. For example, real-time data validation and alerting mechanisms can flag out-of-spec results, missing values, or deviations from defined procedures allowing for immediate intervention before issues escalate.
By enforcing SOP adherence, maintaining version control of test methods, and managing instrument calibration and analyst certifications, a LIMS acts as a compliance partner, not just a data repository.
Ready to transform your lab operations?
Book a demo of Xybion Pharmaceutical LIMS today and discover how it can help your organization ensure compliance, accelerate batch release, reduce human error, and unlock true operational efficiency.