Pharmaceutical LIMS Best Practices: Ensuring Drug Quality and Safety
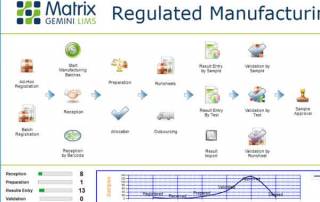
Pharmaceutical LIMS supports drug quality and safety through automation, data integrity, and regulatory compliance. Learn best practices for implementation and key features to look for in a robust, audit-ready solution.