Environmental Monitoring
Simplifies and automates environmental monitoring in QA Labs
Ensuring Safe Manufacturing/Processing Facilities
Collecting samples from different risk areas in and around a production environment provides management the ability to monitor the effectiveness of good hygiene and segregation practices. Through the use of settle plates, air filtration, or sponges/swabs, any microorganisms present in the environment may be captured for analysis.
Matrix Gemini Environmental Monitoring module is perfect for a wide variety of industries including Food and Beverage, Pharmaceutical, Medical Devices, Cosmetics and Healthcare – anywhere where regular testing with swabs, settle plates or air filtration monitoring is used to determine the level of microbial contamination to ensure hygienic/sterile conditions.
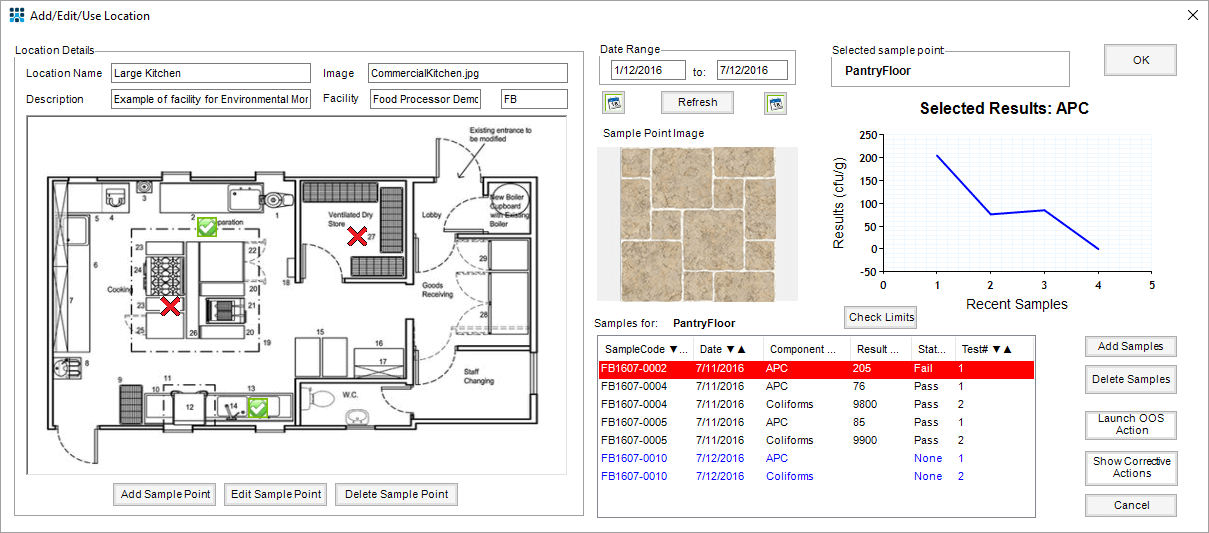
Graphical Mapping to Identify Areas of Concern
The location of sampling sites are captured graphically and overlaid on an image or diagram of the production area being monitored. Multiple areas per facility may be configured, each with multiple sampling points.
The sampling frequency of each sample location can be set independently. The system ensures the correct type of samples are collected according to the defined procedure and appropriate tests assigned. After analysis, the test results are entered so that management can view results by location. Different limits may be set to flag samples when result thresholds for different actions are reached, prompting further investigation by the QA team.


Corrective & Preventive Actions
Fully integrated with Matrix Gemini CAPA Management module. Out Of Specification (OOS) results can trigger the launch of corrective actions. The severity of incident may be classified and responsible personnel assigned for various tasks. Investigative files may be attached for complete documentation.
Users can also view all past corrective actions for tracking and audit purposes. Logging and documenting corrective actions is a key to many quality assurance systems. It allows accurate historic auditing of production sites to meet good practice guidelines and legislative needs. It also helps to drive quality improvement cycles.




